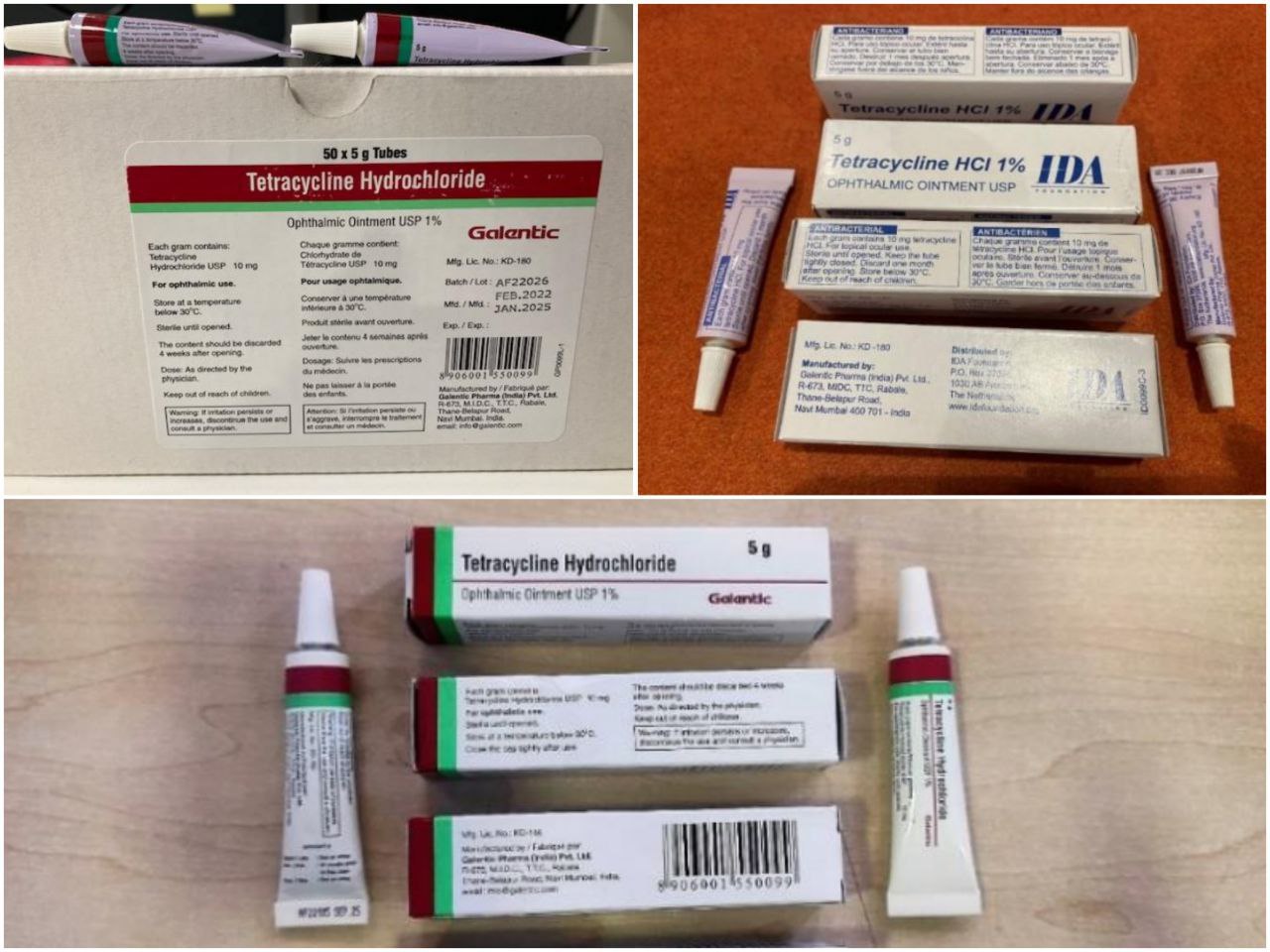
Jahon sog‘liqni saqlash tashkiloti 2023 yil 22 fevral sanasida eʼlon qilgan tibbiy mahsulot to‘g‘risidagi №2/2023-sonli xabarnomasida: Hindistonning "Galentic Pharma Pvt. Ltd." kompaniyasi ishlab chiqargan oftalmologiyada qo‘llaniladigan "Tetratsiklin gidroxloridi USP 1%" surtma dori preparati turli mamlakatlarga har xil markirovka yorlig‘i ostida yetkazib berilgani shuningdek, baʼzi partiyalarda bir qator sifat muammolari boriligini maʼlum qilib, mazkur dori preparatini ehtiyotkorlik yuzasidan qo‘llamasli
More