Mazkur savol yuzasidan Farmatsevtika tarmog‘ini rivojlantirish agentligi direktori maslahatchisi Muhabbat Ibragimova fikrlari bilan qiziqdik.
More

Mazkur savol yuzasidan Farmatsevtika tarmog‘ini rivojlantirish agentligi direktori maslahatchisi Muhabbat Ibragimova fikrlari bilan qiziqdik.
More
O‘zbekiston Respublikasi Sog‘liqni saqlash vazirining 2016 yil 29 iyundagi 60-son buyrug‘i (ro‘yxat raqami 2809-son, 12.07.2016) bilan Tibbiy buyumlar ro‘yxati tasdiqlangan. Mazkur ro‘yxatda 27 to‘plamdagi tibbiy buyumlar jamlanmasi o‘rin olga
More
Olib borilgan ekspertiza hulosasiga ko‘ra, 7731 turdagi 1 319 230 o‘ram sifatsiz, kontrafakt, yaroqlilik muddati tugagan farmatsevtika mahsulotlari tibbiyot amaliyotida qo‘llash mumkin emasligi aniqlandi hamda laboratoriya tahlillari bo‘yicha barcha maʼlumotlar tegishli organlarga taqdim qilindi.
More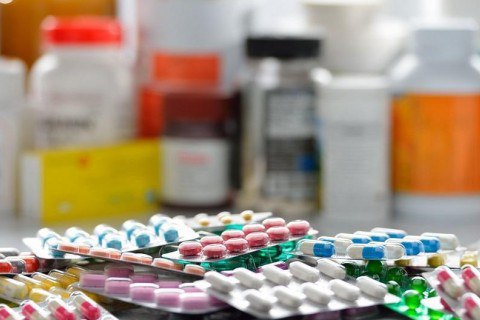
Respublikamizdagi Farmatsevtika mahsulotlarini sertifikatlashtirish organlariga bo‘lgan murojaatlar asosida 2022 yil davomida taqdim qilingan dori vositalari belgilangan tartibda sertfikatlashtirish uchun tekshiruvdan o‘tkazildi.
More
"Dori vositalari, tibbiy buyumlar va tibbiy texnika ekspertizasi va standartlashtirish Davlat markazi"ning Qiyoslash laboratoriyasi tomonidan 2022 yil davomida qiyoslash sohasida amalga oshirilgan ishlar sarhisob qilindi.
More
Mamlakatimizda Prezidentimiz rahbarligida tinchlikni mustahkamlash, xalqimiz farovonligini taʼminlash, keksalarni eʼzozlash, ertamiz egalarini barkamol etib voyaga yetkazishga davlat siyosatining ustuvor yo‘nalishi sifatida alohida eʼtibor qaratilayotganligi taʼkidlash joiz. Binobarin, buyuk ajdodlarga munosib avlod bo‘lish, yurtimiz taraqqiyotiga daxldorlik hissi bilan yashash har birimizning, ayniqsa, yoshlarning muqaddas burchidir.
More
Ijtimoiy tarmoqlarda Samarqand shahridagi dorixonalardan xarid qilingan “Dok-1 Maks” tabletka va sirop dori vositalarini qabul qilgandan so‘ng bemorlarda turli nojo‘ya taʼsirlar kuzatilgani haqida xabarlar tarqaldi.
More
Joriy yilning 20 dekabr kuni “O‘zbekiston akkreditatsiya markazi” DK tomonidan ILAC MRA va IAF MLA ko‘p tomonlama tan olish bitimlariga qo‘shilishi natijalari va baholovchi hamda texnik ekspertlarning 2022 yilgi faoliyati yakunlari bo‘yicha anjuman tashkil etildi.
More
So‘ngi yillarda yurtimizda metrologiya sohasida keng qamrovli islohotlar amalga oshirilmoqda. Kundan kunga bu soha yana-da tez rivojlanib, tobora takomillashib bormoqda.
More
Joriy yilning 20 oktyabr va 15 noyabr kunlari Andijon va Samarqand viloyatida Dori vositalari tibbiy buyumlar va tibbiy texnika ekspertizasi va standartlashtirish Davlat markazi mutaxassislari tomonidan Vazirlar Mahkamasining tegishli qarori bilan bosqichma-bosqich joriy etilayotgan, dori vositalari va tibbiy buyumlarini majburiy raqamli markirovkalash tizimi va uning ahamiyati mavzusida navbatdagi uchrashuv tashkil qilindi.
More